

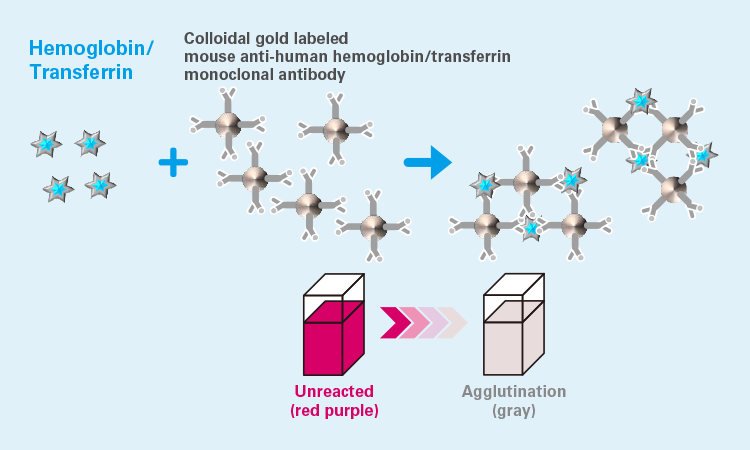
The conventional fecal occult blood test uses a chemical to perform the test, such as guaiac test (g-FOBT). However, our FIT Hemoglobin and Trasnferrin products use an immunoassay that reacts specifically to human hemoglobin/transferrin, eliminating the need for dietary restrictions before the test. In addition, our products use a colloidal gold agglutination method that optically measures color and turbidity changes caused by antigen-antibody reactions at two different wavelengths, and they are highly accurate and sensitive.
Measurement principle: Colloidal gold agglutination1)
The stability of hemoglobin was excellent in the Specimen Collection Container A, and after sampling, the samples (50–381 ng/mL) remained negative or positive (cut-off value: 100 ng/mL) for 33 days at -40℃ and 7℃, for 14 days at 25℃, and for 7 days at 37℃.2)
Hemoglobin stability after sampling2)
| -40°C, for 33 days | 7°C, for 33 days | 25°C, for 14 days | 37°C, for 7 days | |
|---|---|---|---|---|
| 50 ng/mL | > 90% | > 90% | > 90% | > 90% |
| 169 ng/mL | > 90% | > 90% | > 90% | > 70% |
| 266 ng/mL | > 90% | > 90% | > 70% | > 50%* |
| 381 ng/mL | > 90% | > 90% | > 50%* | > 30%* |
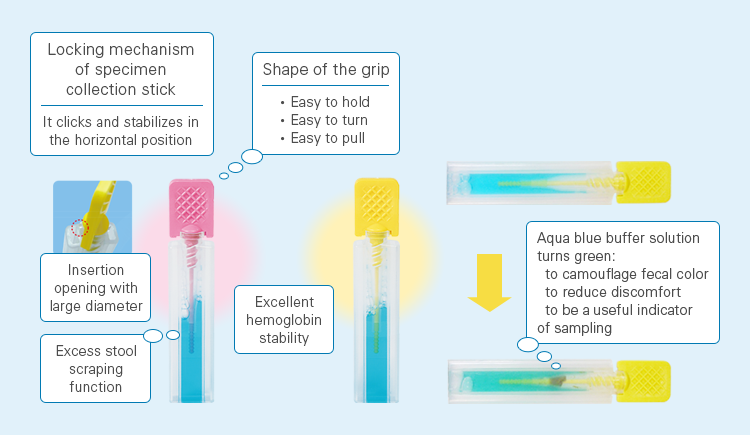
The Specimen Collection Container A is flat and rectangular in shape and easy to handle, and has high structural strength1) to withstand shocks during transportation. After fecal sampling, the aqua blue buffer solution turns green, which camouflages the fecal color and eases the user’s discomfort and serves as a useful indicator to show that the user has taken a fecal sample.
Features of Specimen Collection Container A1)
The efficacy of the fecal immunochemical test (FIT) has been established. However, hemoglobin, the substance to be measured, may become inactivated by the bacteria in the large intestine if it remains in the large intestine for a long time, and this instability may result in false negatives. Transferrin in the stool is one of the blood components and is relatively stable against the bacteria in the large intestine. Accordingly, measuring transferrin simultaneously with hemoglobin provides more accurate information on bleeding in the large intestine and decreases the risk of oversight due to false negative with hemoglobin.3) This simultaneous assay can be expected to show higher accuracy and efficacy in colorectal cancer screening.4)
References
Scroll horizontally to see more.
Leaflet of FIT Hemoglobin, FIT Transferrin and NESCAUTO® Cp Auto using NS-Prime®
Performance data of FIT Hemoglobin and FIT Transferrin NS-Prime*
Performance data of FIT Hemoglobin and FIT Transferrin AA01*