

The conventional fecal calprotectin test often uses the ELISA method. However, our NESCAUTO® Cp Auto products use a colloidal gold agglutination method, which is an immunoassay. The gold colloid agglutination method optically measures the color change caused by antigen-antibody reactions at two different wavelengths, and therefore, it is highly accurate and sensitive.1)
Measurement principle: Colloidal gold agglutination2)
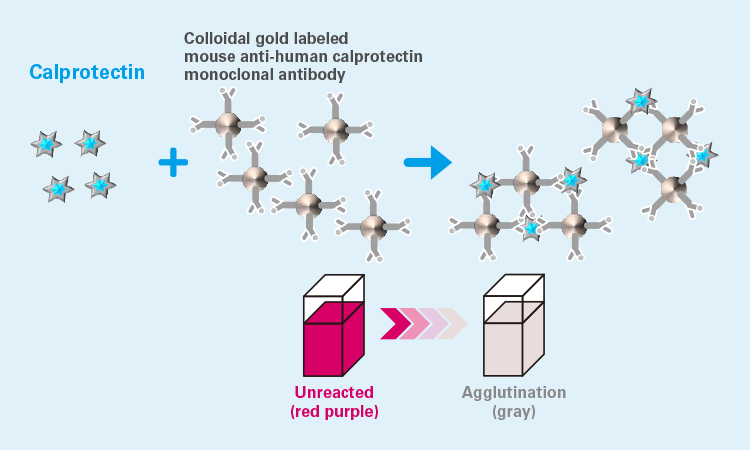
Compared to the conventional ELISA method, our NESCAUTO® Cp Auto products do not require sample preparation, which enables easy and quick use. The measurement time is as short as 10 to 15 minutes, allowing both the patient and the doctor to discuss and consult on the treatment policy together (shared decision making) after the patient is informed of the results on the day of the test, which is also beneficial as it leads to proactive treatment (improving adherence).
Not only calprotectin but also hemoglobin in the fecal samples can be measured simultaneously from a sample obtained in a single Specimen Collection Container A, and therefore, disease activity can be assessed from the perspectives of both inflammation (calprotectin) and bleeding (hemoglobin).3)
A strong correlation has been observed between the fecal calprotectin levels measured using our NESCAUTO® Cp Auto products and those measured using the conventional ELISA method (r = 0.98, p < 0.01). And, a strong correlation was observed in the colonoscopy findings [r=0.70, p < 0.01 for ulcerative colitis (UC); r=0.58, p < 0.01 for Crohn’s disease (CD)].4) In addition, high sensitivity and specificity can be expected for both UC and CD.4)
References
Scroll horizontally to see more.
Leaflet of FIT Hemoglobin, FIT Transferrin and NESCAUTO® Cp Auto using NS-Prime®